Mechanism of Action (MOA) for the First FDA-Approved Treatment for Cholestatic Pruritus in Alagille Syndrome1
Mechanism of Disease (MOD)
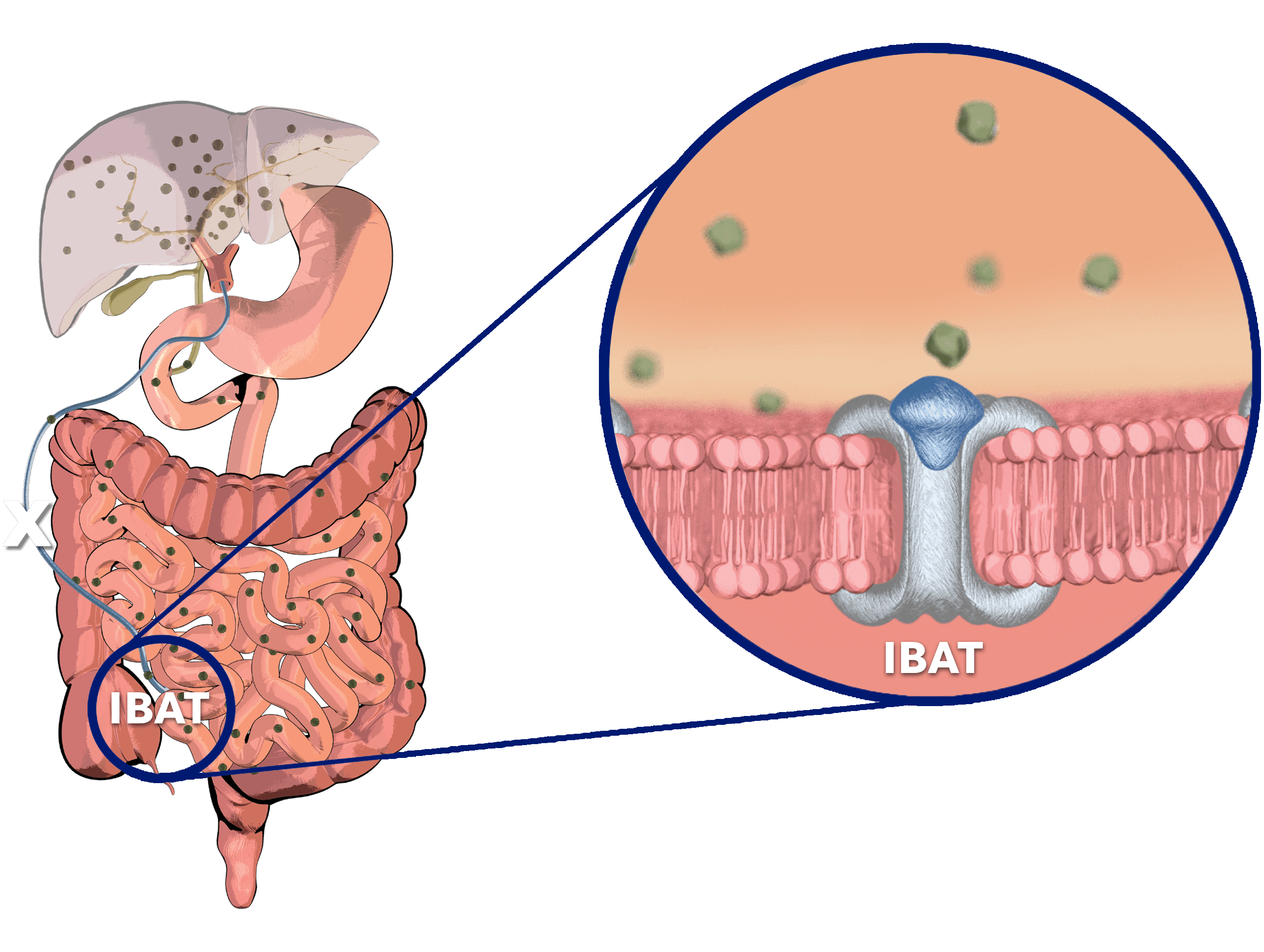
Bile Acid Buildup in Alagille Syndrome
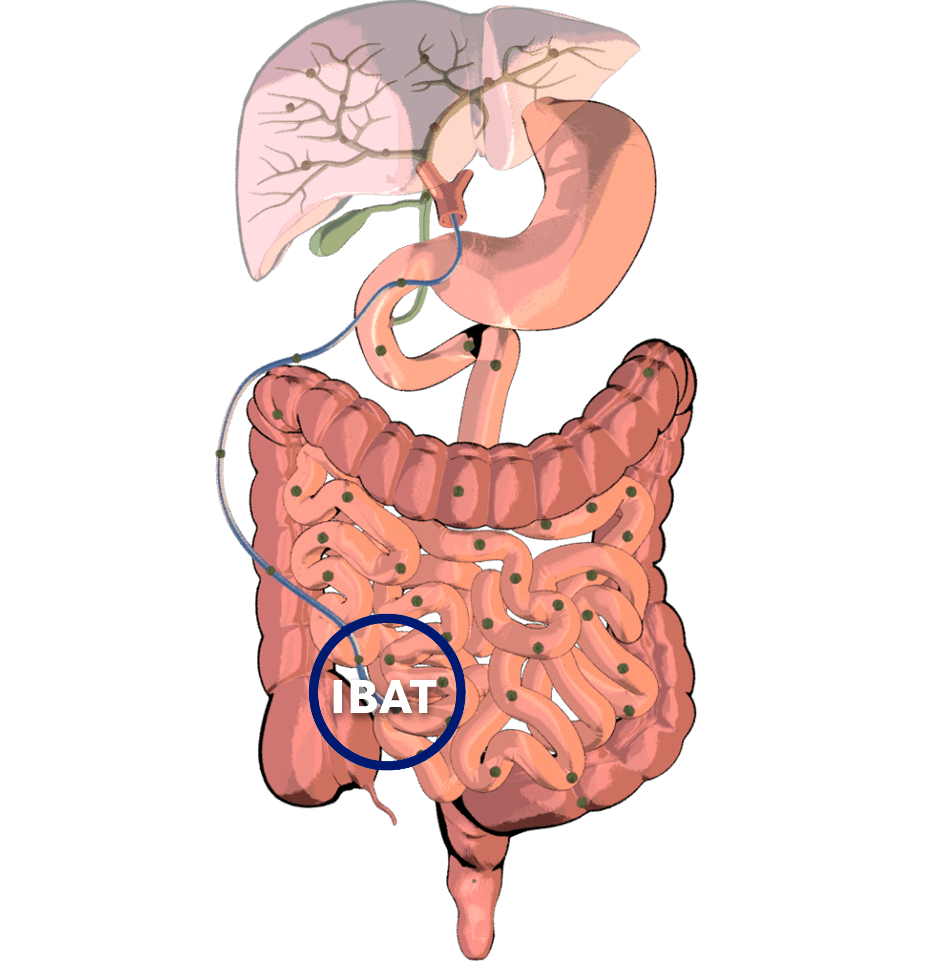
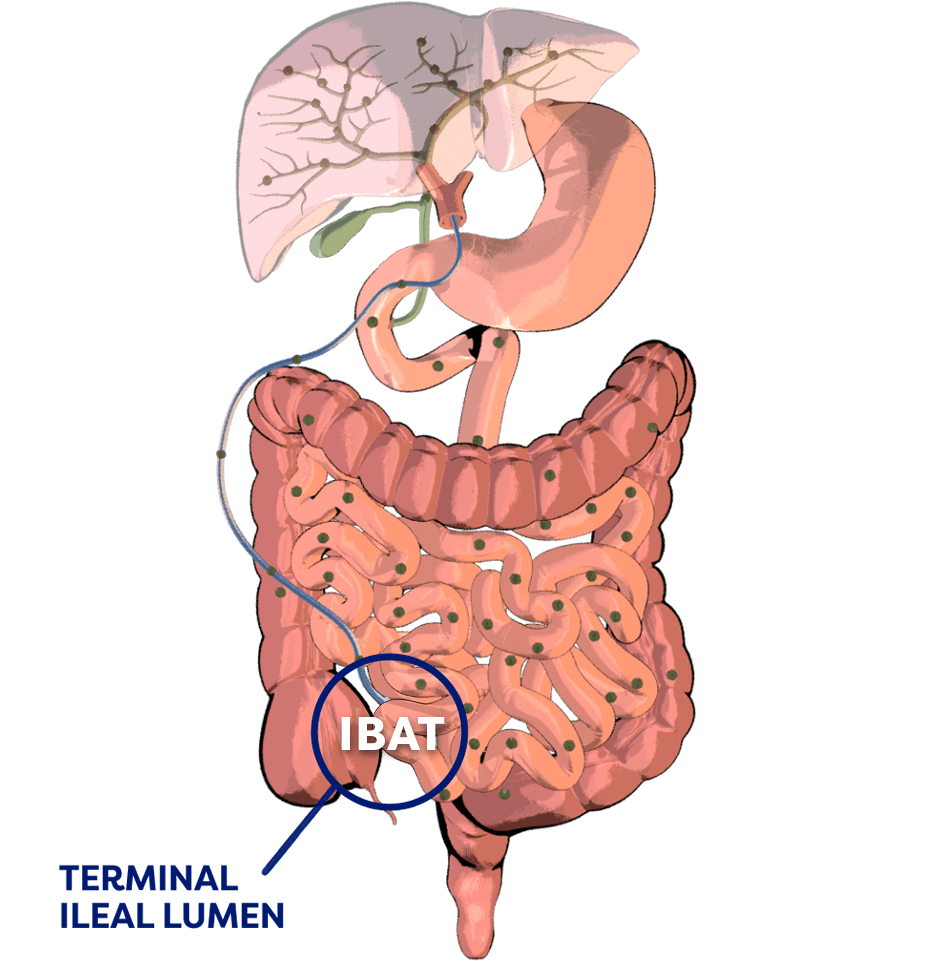


Normally, adequate enterohepatic circulation is crucial for homeostatic maintenance of the body’s bile acid pool.2
- This process starts with bile acid synthesis in hepatocytes and its subsequent biliary secretion, primarily mediated by the bile salt export pump (BSEP)2
- Bile acids then move through bile ducts to the gallbladder for storage2
- Later, bile acids are released into the small intestine to aid in lipid digestion and absorption2

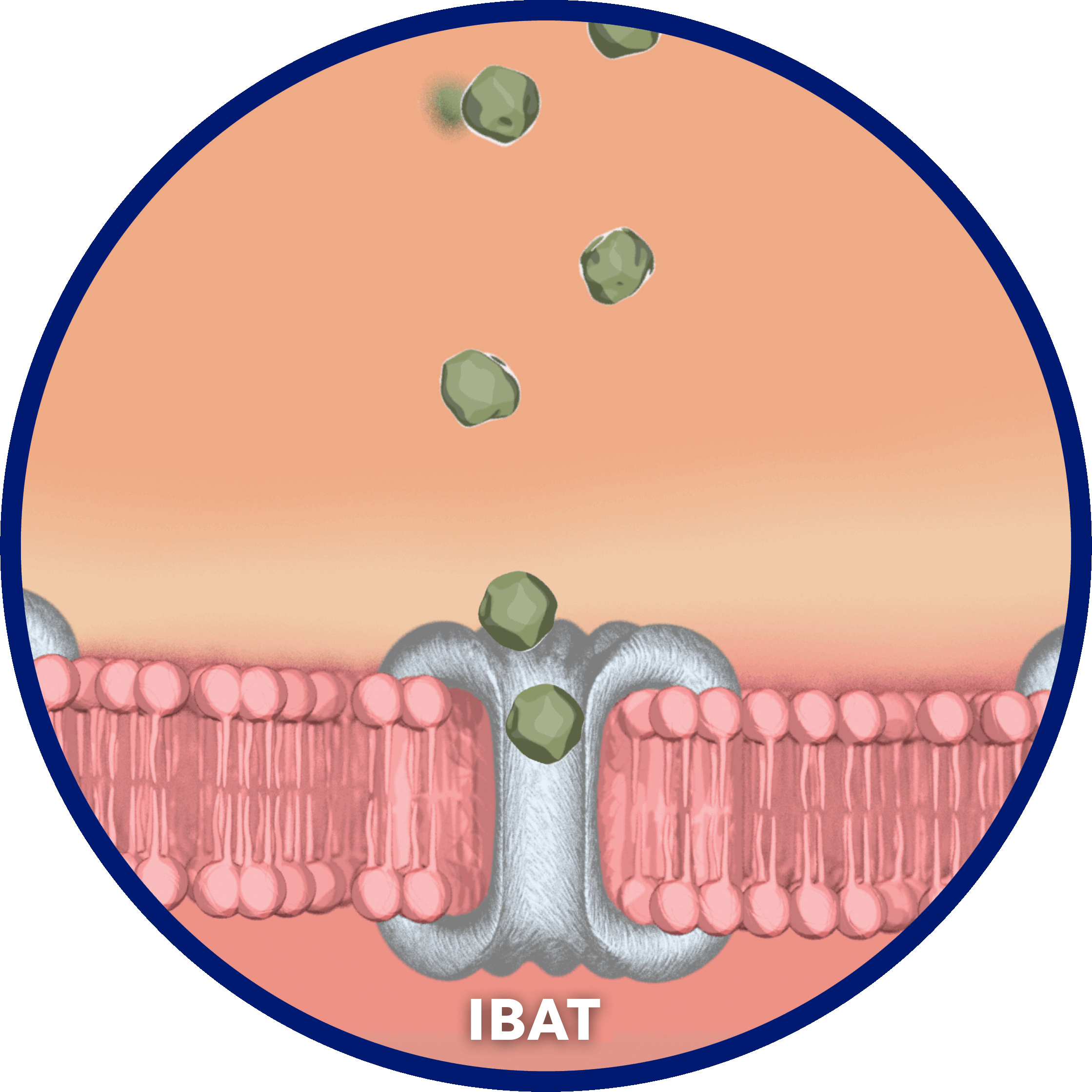
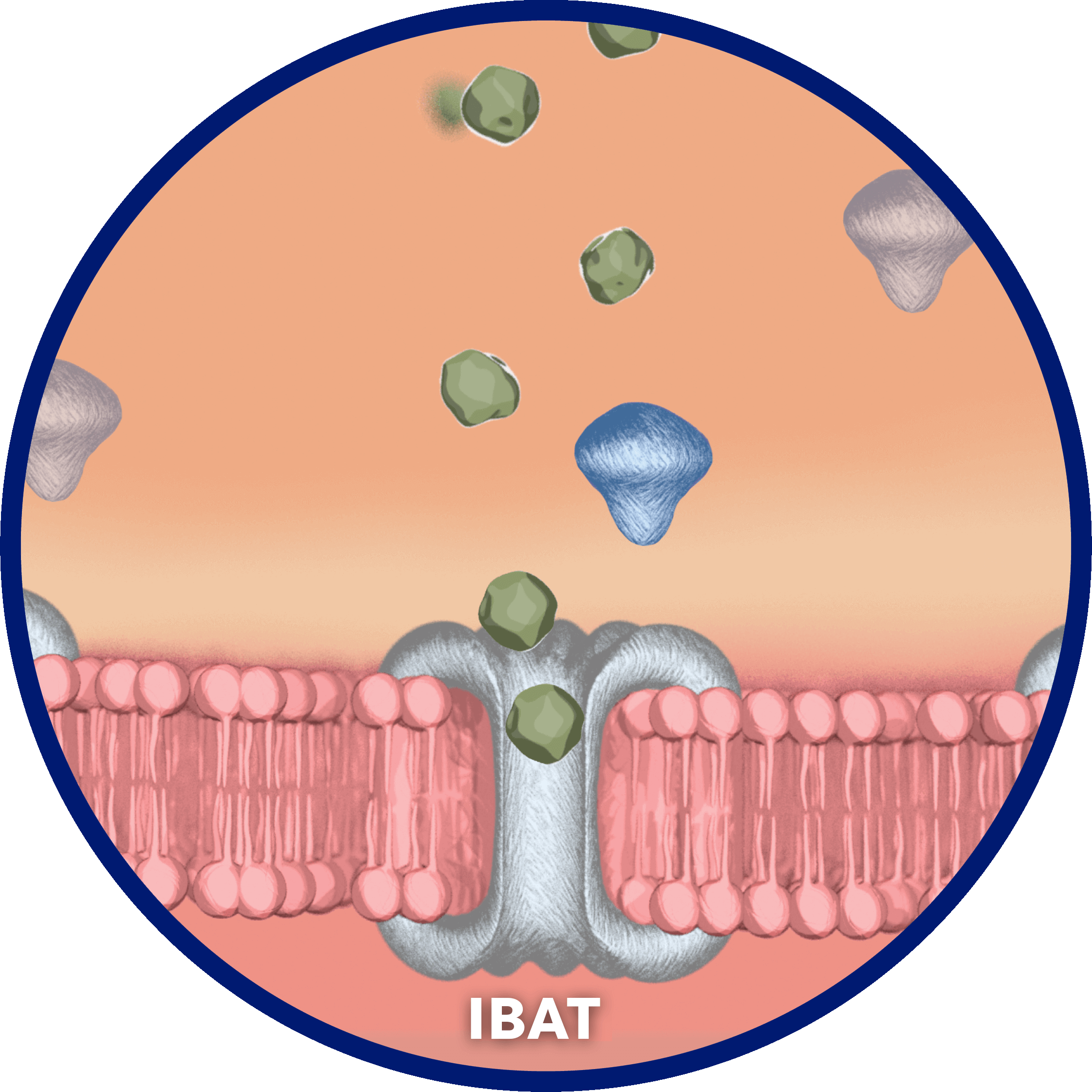
Per cycle, approximately 95% of bile acids are reabsorbed…
…by the ileal bile acid transporter (IBAT) in the terminal ileal lumen for return to the liver through the hepatic portal vein.2

Bile acids that are not reabsorbed from the intestine via the IBAT are lost in feces…
…and, under steady-state conditions, are replaced by hepatic de novo synthesis.2

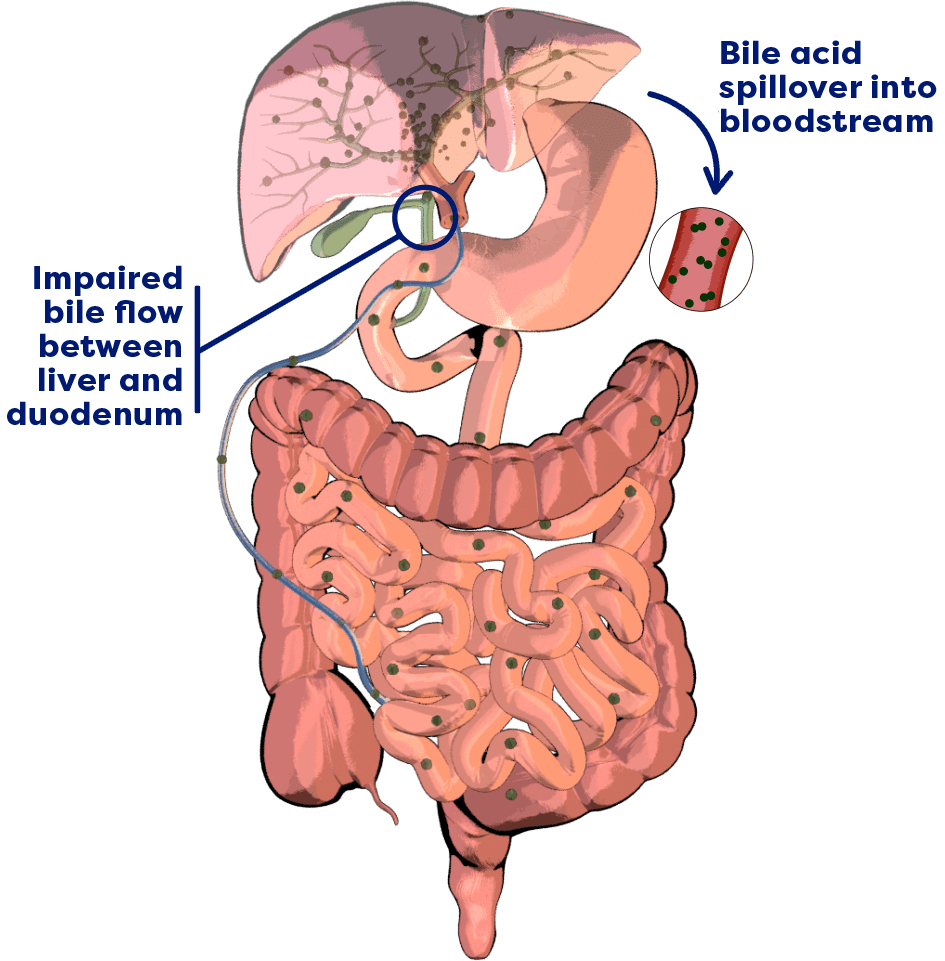
In cholestatic liver disease, bile flow is impaired between the liver and the duodenum, leading to a buildup of bile acids in the liver.3,4
There is also spillover of bile acids into the bloodstream, resulting in an increase in serum bile acids (sBA) systemically.3,4

In Alagille syndrome, bile ducts are narrow, malformed, and/or reduced in number.2,5
This results in bile acid buildup in the liver and bloodstream.2,5
Related to elevated serum bile acid (sBA) levels, cholestatic pruritus in Alagille syndrome is among the worst of any cholestatic liver disease.2,6-8
Narrator [00:00:00]
Let's take a closer look at bile acid buildup in Alagille syndrome. In normal anatomy, adequate enterohepatic circulation is crucial for homeostatic maintenance of the body's bile acid pool. This process starts with bile acid synthesis in hepatocytes and their subsequent biliary secretion, primarily mediated by the bile salt export pump, or BSEP. Bile acids then move through bile ducts to the gallbladder for storage and, later, for
[00:00:30]
release into the small intestine to aid in lipid digestion and absorption.
Per cycle, approximately 95% of bile acids are reabsorbed by the ileal bile acid transporter, or IBAT, in the terminal ileal lumen for return to the liver through the hepatic portal vein. Bile acids that are not reabsorbed from the intestine via the IBAT are lost in feces, and, under steady-state conditions, are replaced by hepatic de novo synthesis. In cholestatic liver disease, the flow of bile is impaired,
[00:01:00]
leading to a buildup of bile acids in the liver. There is also spillover of bile acids into the bloodstream, resulting in an increase in serum bile acids systemically.
In Alagille syndrome, narrow, malformed, and/or a reduced number of bile ducts results in bile acid buildup in the liver and bloodstream. Symptoms associated with elevated serum bile acids may include debilitating cholestatic pruritus, which is considered to be the most troublesome symptom of Alagille syndrome. Accumulation of toxic serum bile
[00:01:30]
acids may also lead to liver damage. With LIVMARLI, you can arm patients to battle bile acid buildup. A treatment for patients with cholestatic pruritus in Alagille syndrome who are 3 months of age and older…
[music]
Narrator: …and the first FDA-approved treatment for cholestatic pruritus in Alagille syndrome. LIVMARLI helps battle bile acid buildup by inhibiting the IBAT.
[00:02:00]
By interrupting the recirculation of bile acids to the liver, LIVMARLI increases the excretion of bile acids in feces in the terminal ileum, and decreases the bile acid pool in the body. Although the complete way by which LIVMARLI improves cholestatic pruritus in patients with Alagille syndrome is unknown, it may involve inhibition of the IBAT, which results in decreased reuptake of bile salts, as observed by a decrease in serum bile acid levels.
[00:02:30]
In the ICONIC pivotal study, serum bile acid reductions correlated with reductions in cholestatic pruritus intensity, further supporting the potential causal relationship between the two.
INDICATION
LIVMARLI is indicated for the treatment of cholestatic pruritus in patients who are 3 months of age and older with Alagille syndrome.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
LIVMARLI is contraindicated in patients with prior or active hepatic decompensation events (eg, variceal hemorrhage, ascites, or hepatic encephalopathy).
WARNINGS AND PRECAUTIONS
Hepatotoxicity: LIVMARLI treatment is associated with a potential for drug-induced liver injury. In the Alagille syndrome trial, treatment-emergent elevations of liver tests or worsening of liver tests occurred.
Obtain baseline liver tests and monitor during treatment. Liver-related adverse reactions and physical signs of hepatic decompensation should also be monitored. Dose reduction or treatment interruption may be considered if abnormalities occur in the absence of other causes. For persistent or recurrent liver test abnormalities, consider treatment discontinuation. Permanently discontinue LIVMARLI if a patient experiences the following: persistent or recurrent liver test abnormalities, clinical hepatitis upon rechallenge, or a hepatic decompensation event.
Gastrointestinal (GI) Adverse Reactions: Diarrhea and abdominal pain were reported as the most common adverse reactions. Monitor for dehydration and treat promptly. Consider reducing the dosage or interrupting dosing if a patient experiences persistent diarrhea or has diarrhea with bloody stool, vomiting, dehydration requiring treatment, or fever.
Fat-Soluble Vitamin (FSV) Deficiency: Patients can have FSV deficiency (vitamins A, D, E, and K) at baseline, and LIVMARLI may adversely affect absorption of FSVs. If bone fractures or bleeding occur, consider interrupting LIVMARLI and supplement with FSVs. LIVMARLI can be restarted once FSV deficiency is corrected and maintained at corrected levels.
Risk of Propylene Glycol Toxicity (Pediatric Patients Less Than 5 Years of Age): Total daily intake of propylene glycol should be considered for managing the risk of propylene glycol toxicity. Monitor patients for signs of propylene glycol toxicity. Discontinue LIVMARLI if toxicity is suspected.
ADVERSE REACTIONS
The most common adverse reactions are diarrhea, abdominal pain, vomiting, FSV deficiency, liver test abnormalities, and bone fractures.
DRUG INTERACTIONS
Administer bile acid binding resins at least 4 hours before or 4 hours after administration of LIVMARLI. A decrease in the absorption of OATP2B1 substrates (eg, statins) due to OATP2B1 inhibition by LIVMARLI in the GI tract cannot be ruled out. Consider monitoring the drug effects of OATP2B1 substrates as needed.
DOSING INFORMATION
LIVMARLI should be taken 30 minutes before a meal. The provided oral dosing dispenser must be used to accurately measure the dose. Any remaining LIVMARLI should be discarded 100 days after first opening the bottle.
[music]
Narrator: [00:06:00]
It's time for your patients with Alagille syndrome to take aim against bile acid buildup. It's time for LIVMARLI.[00:06:11] [END OF AUDIO]
SEE THE LIVMARLI MECHANISM OF ACTION (MOA)—IN ACTION


Mechanism of Action
LIVMARLI Helps Battle Bile Acid Buildup


LIVMARLI is an ileal bile acid transporter (IBAT) inhibitor and the first approved treatment for cholestatic pruritus in patients with Alagille syndrome who are 3 months of age and older.1

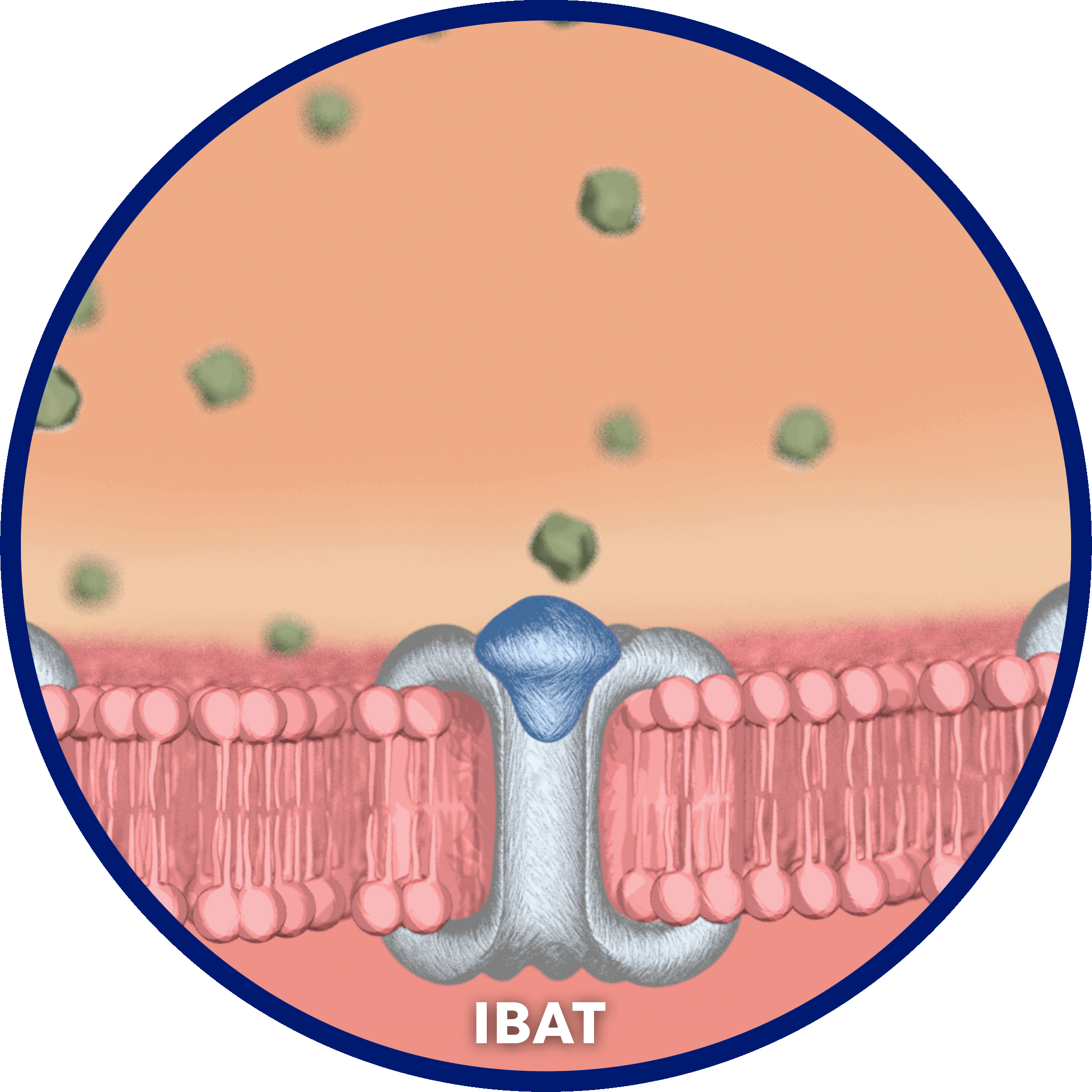
LIVMARLI interrupts recirculation of bile acids to the liver and increases fecal excretion of bile acids to decrease the bile acid pool in the body, with minimal systemic absorption.1,9,10

Although the complete mechanism by which LIVMARLI improves cholestatic pruritus in patients with Alagille syndrome is unknown, it may involve inhibition of the ileal bile acid transporter (IBAT), which results in decreased reuptake of bile salts, as observed by a decrease in sBA.1
- In the ICONIC study, sBA reductions correlated with reductions in cholestatic pruritus intensity, further supporting the potential causal relationship between the two11

During the first year of treatment, 83% (n=24/29) of patients with Alagille syndrome experienced a ≥20% reduction in sBA levels vs baseline with LIVMARLI.9,12
Early Improvements With Long-Term Impact
Significant improvements in cholestatic pruritus from baseline were seen at the very first assessment.9 For those who were treated with LIVMARLI in 3 long-term clinical trials (N=76) as part of a post hoc analysis, 93% of patients remained transplant free 6 years after starting LIVMARLI.13,14*
See the Efficacy Data
Encourage patients to download the Itch✓ app to help them track symptom patterns over time and generate customized reports to share at appointments.
Check Out the Itch✓ App
Mirum Access Plus assists both you and your patients at every turn, helping you navigate the payer approval process—and beyond—with ease.
Learn More AboutMirum Access Plus
*Transplant-free survival was defined as time to liver transplant or death.13

