Early Improvements in Cholestatic Pruritus With Long-Term Impact

Cholestatic Pruritus Efficacy
Early Improvements in Cholestatic Pruritus
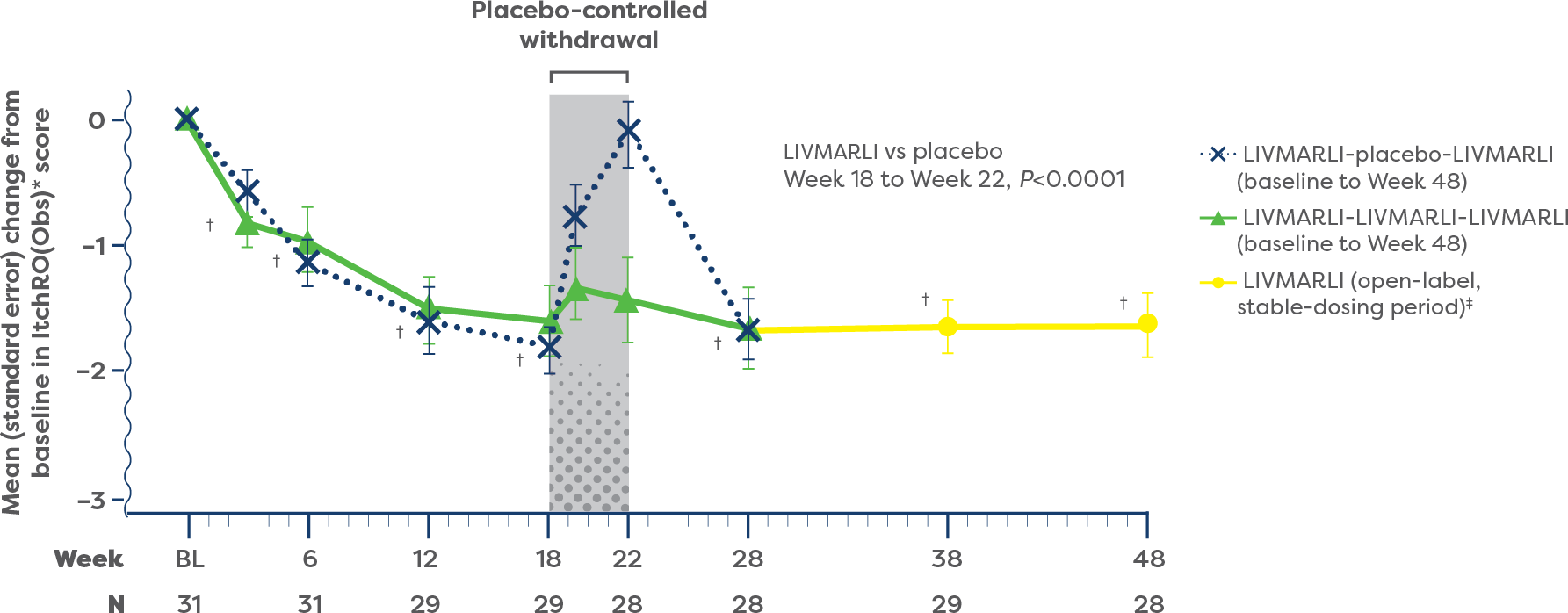
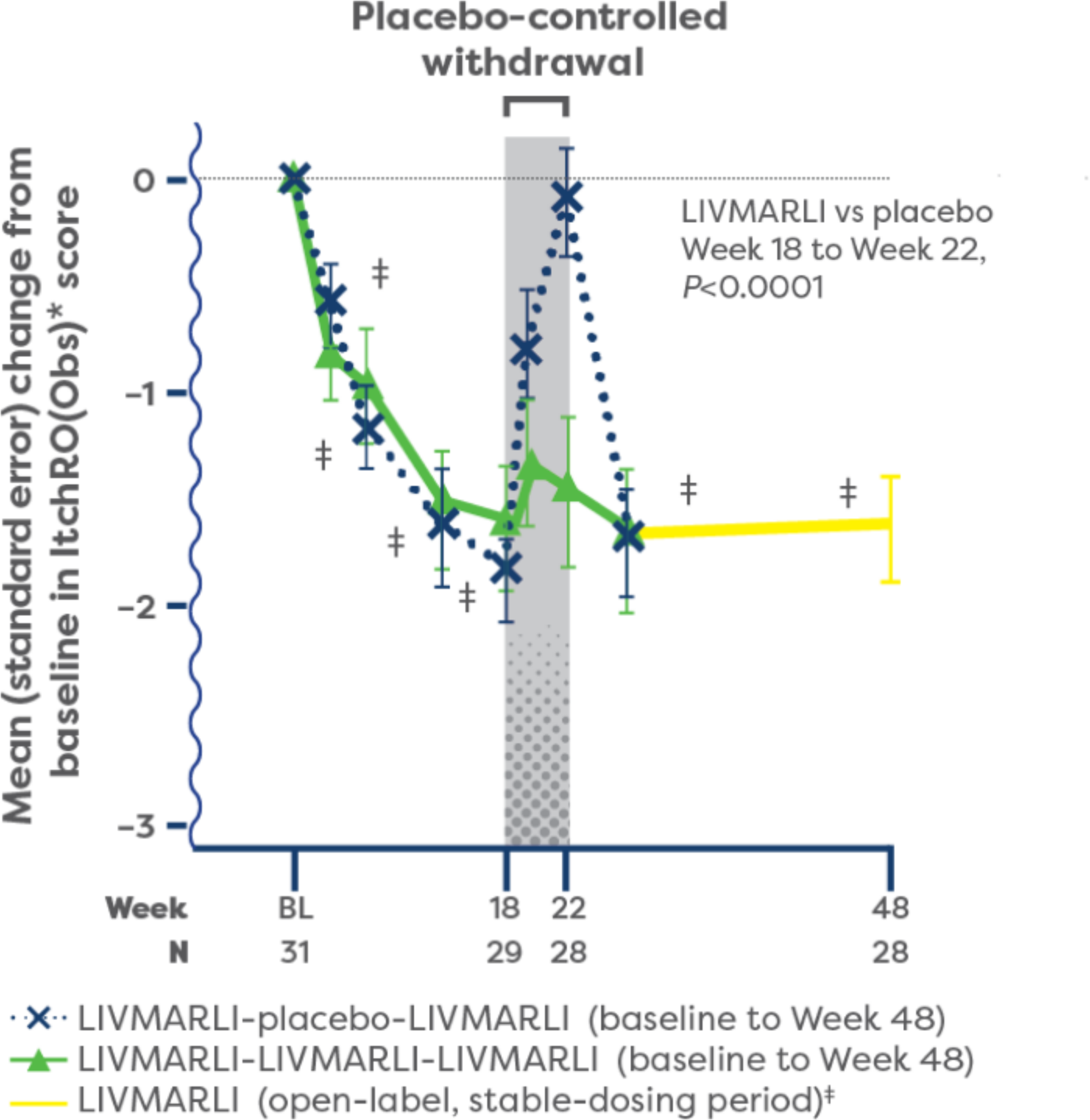
Significant improvements in cholestatic pruritus from baseline were seen at the very first assessment (Week 3), with the full effect achieved by Week 18 and maintained through 1 year with once-daily LIVMARLI (P<0.0001).1,2
For patients who remained on treatment with LIVMARLI in the open-label extension (n=15), cholestatic pruritus responses compared with baseline were durable through nearly 4 years.1
IMPROVEMENTS IN CHOLESTATIC PRURITUS OVER TIME1,2
View Study Design and Select Baseline Characteristics
Study Design
ICONIC is the first pivotal study of an IBAT* inhibitor in Alagille syndrome to demonstrate significant improvement in cholestatic pruritus1†
The ICONIC study assessed efficacy and safety of treatment with LIVMARLI in children ≥1 year old with cholestatic pruritus associated with Alagille syndrome.1,2
This study consisted of an 18-week open-label treatment period; a 4-week randomized, double-blind, placebo-controlled drug-withdrawal period; a subsequent 26-week open-label treatment period; and a long-term open-label extension period.1,2
Cholestatic pruritus responses to LIVMARLI were assessed through approximately 4 years.1

Cholestatic pruritus was assessed at baseline using the Itch Reported Outcome (ItchRO) scale. The ItchRO score is a 0–4 scale, where 0 is none and 4 is very severe.1 This scale takes itch-related symptoms into consideration, including patients’ skin damage, sleep, and irritability.1,3,4
- Participants’ baseline scores were calculated as the average of the daily ItchRO scores over the 2 consecutive weeks during the screening period1
SELECT BASELINE CHARACTERISTICS1
| All Participants (N=31) | |
| Mean age at baseline visit, years (SD) | 5.4 (4.2) |
| Sex, n (%) | — |
| Female | 12 (39) |
| Male | 19 (61) |
| Genotyped mutation within JAG1, n (%) | 31 (100) |
| History of receiving treatment for pruritus, n (%) | — |
| Any medication | 29 (94) |
| Ursodeoxycholic acid | 25 (81) |
| Rifampicin | 23 (74) |
| Naltrexone | 1 (3) |
| Sertraline | 1 (3) |
| Trial parameter, mean (SD) | — |
| ItchRO(Obs) weekly morning average severity score‖ | 2.9 (0.5) |
| CSS score | 3.3 (0.9) |
| sBA, µmol/L | 283 (211) |
*IBAT=ileal bile acid transporter.
†Mean difference –1.4 points [95% CI, –2.1, –0.8].1
‡Included a 6-week dose escalation period for all participants during the first 6 weeks of the open-label treatment period and for participants who received placebo during the RWD.
§Twice per day dosing was allowed after Week 100. The approved dosage of LIVMARLI is 380 mcg/kg once daily.
‖Average ItchRO(Obs) scores are based on the 7 days prior to baseline visit.
FSV supplements were available as SOC throughout the study. No changes beyond SOC in supplementation occurred during the study.1
Safety, tolerability, and pharmacokinetics of LIVMARLI in patients aged 3 months to 1 year were evaluated in RISE, a 13-week, open-label, phase 2 study (N=8). Participants received LIVMARLI 380 µg/kg once daily, in addition to standard of care.2,5
CSS=Clinician Scratch Scale; FSV=fat-soluble vitamin; ItchRO=Itch Reported Outcome; JAG1=jagged canonical Notch ligand 1; RWD=randomized withdrawal period; sBA=serum bile acid; SD=standard deviation; SOC=standard of care.
References: 1. Gonzales E, Hardikar W, Stormon M, et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): a randomised phase 2 study. Lancet. 2021;398(10311):1581-1592. doi:10.1016/S0140-6736(21)01256-3 2. LIVMARLI® (maralixibat) oral solution. Prescribing Information. Mirum Pharmaceuticals, Inc. 3. Kamath BM, Abetz-Webb L, Kennedy C, et al. Development of a novel tool to assess the impact of itching in pediatric cholestasis. Patient. 2018;11(1):69-82. doi:10.1007/s40271-017-0266-4 4. Kamath BM, Spino C, McLain R, et al. Unraveling the relationship between itching, scratch scales, and biomarkers in children with Alagille syndrome. Hepatol Commun. 2020;4(7):1012-1018. doi:10.1002/hep4.1522 5. Gonzales E, Jankowska I, Horslen S, et al. Safety and tolerability characterization of maralixibat in infants with ALGS from 2 months of age: interim results from the RISE study. Poster presented at: American Association for the Study of Liver Diseases: The Liver Meeting; November 4-8, 2022; Washington, DC.

During the first year of treatment, 84% (n=26/31) of patients with Alagille syndrome experienced clinically meaningful improvements in cholestatic pruritus compared with baseline with once-daily LIVMARLI.1
- “Clinically meaningful” was defined as ≥1-point ItchRO(Obs) improvement vs baseline1
At 1 year, improvements in cholestatic pruritus were correlated with decreases in serum bile acid (sBA) (r=0.47).3
POST HOC ANALYSIS:
During the first year, patients receiving LIVMARLI in the pivotal ICONIC study had an increasing proportion of days with minimal to no cholestatic pruritus.4
- In patients who remained on LIVMARLI (n=21) during the open-label extension (beyond 48 weeks), the median proportion of days with minimal to no cholestatic pruritus was 95%4§
*Cholestatic pruritus was assessed each day, in the morning and evening, using the Itch Reported Outcome (ItchRO) scale—a validated tool designed to assess the impact of cholestatic pruritus in people with cholestatic liver disease, including Alagille syndrome. The ItchRO score is a 0–4 scale, where 0 is none, 1 is mild, 2 is moderate, 3 is severe, and 4 is very severe. Changes in ItchRO score of 1.0 or more have been shown to be clinically meaningful. ItchRO(Obs) was completed by caregivers and was the basis for the key pruritus endpoint. The patient-rated ItchRO (ItchRO[Pt]) was completed independently by participants aged 9 years or older and with caregiver assistance for participants aged 5 to 8 years.1
†Change from baseline, P<0.0001.2
‡Included an initial 6-week dose escalation for participants previously receiving placebo.1
§Based on mean daily morning ItchRO(Obs) scores in patients who remained on LIVMARLI through 4 years.4
Cholestatic Pruritus Improvements
Long-Term Liver Impact
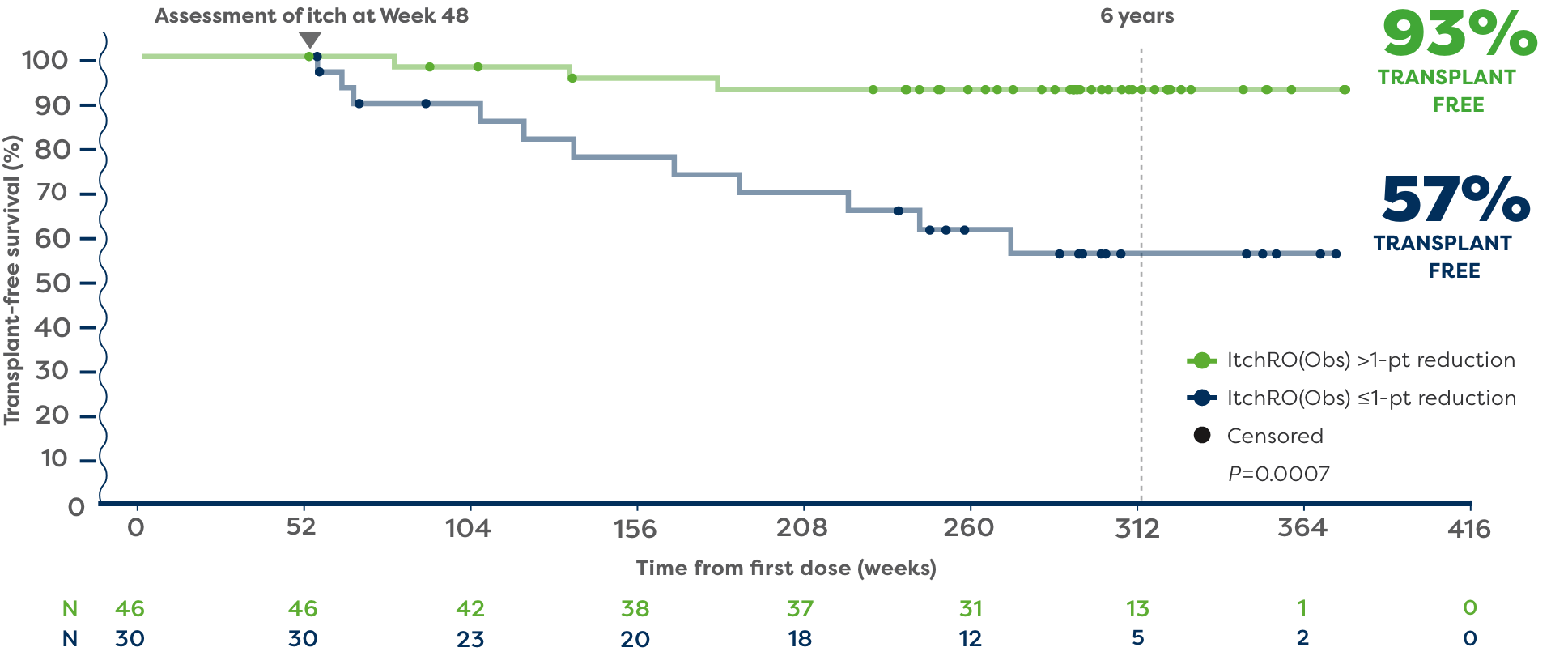
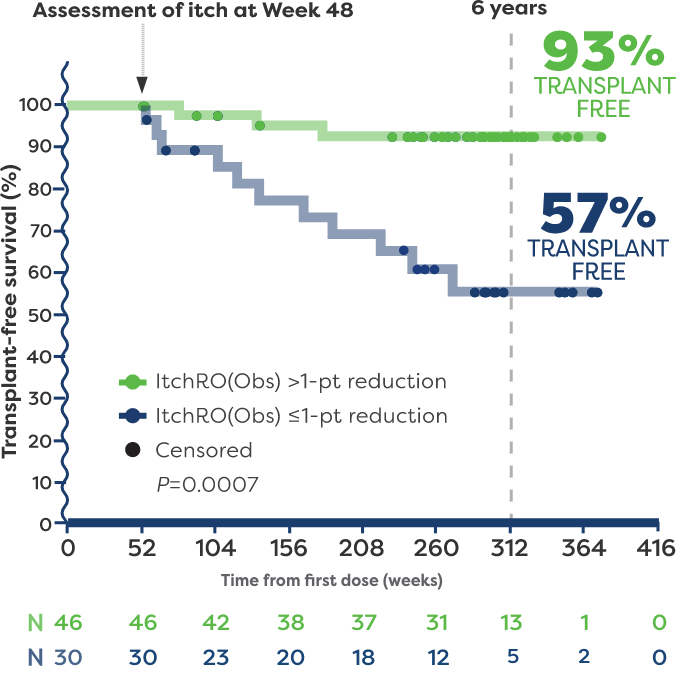
In a post hoc analysis, patients with Alagille syndrome who were treated with LIVMARLI in 3 long-term clinical trials were followed to identify predictors of long-term, transplant-free survival.5
In the post hoc analysis, 93% of patients who achieved a >1-point reduction in ItchRO(Obs) (n=46) remained transplant free 6 years after starting LIVMARLI.5,6*†
57% of patients who had ≤1-point reduction in ItchRO(Obs) (n=30) remained transplant free 6 years after starting LIVMARLI.5,6
*The impact of LIVMARLI treatment on transplant-free survival has not been established. No liver histology to assess hepatic fibrosis was collected.
†Transplant-free survival was defined as time to liver transplant or death.5
Refractory cholestatic pruritus was an indication for liver transplant in a large percentage of patients with Alagille syndrome.5
Hide Study Design
In a post hoc analysis, patients with Alagille syndrome who were treated with LIVMARLI in 3 long-term clinical trials (N=76) were followed to identify predictors of long-term, transplant-free survival.1
- Transplant-free survival was defined as time to liver transplant or death1
- Median follow-up was 5.1 years (range: 1.0 year to 7.3 years)2
- This analysis included patients aged 14 months to 17.25 years, with median serum bile acids (sBA) 184 µmol/L (IQR, 78 to 361) and median ItchRO(Obs) score 2.7 (IQR, 2.1 to 3.1) at baseline1
- Patients (N=76) with moderate-to-severe cholestatic pruritus who had a perceived benefit from LIVMARLI, remained on treatment for at least 48 weeks, and had lab results at 48 weeks were included in this analysis. No placebo arm was included1
References: 1. Sokol RJ, Gonzales EM, Kamath BM, et al. Predictors of 6-year event-free survival in Alagille syndrome patients treated with maralixibat, an ileal bile acid transporter inhibitor. Hepatology. Published June 7, 2023. 2. Sokol J, Gonzales E, Kamath BM, et al. Predictors of 6-year event-free survival in patients with Alagille syndrome treated with maralixibat, an IBAT inhibitor. Paper presented at: European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN): Annual Meeting; June 22-25, 2022; Copenhagen, Denmark.
ItchRO
Cholestatic pruritus was assessed using the Itch Reported Outcome (ItchRO[Obs]) tool. The ItchRO score is a 0–4 scale, where 0 is none and 4 is very severe.7
Always
Safety First!
LIVMARLI has a well-characterized safety and tolerability profile for cholestatic pruritus in patients with Alagille syndrome.8,9
See the Safety Profile
Encourage patients to download the Itch✓ app to help them track symptom patterns over time and generate customized reports to share at appointments.
Check Out the Itch✓ App
Mirum Access Plus assists both you and your patients at every turn, helping you navigate the payer approval process—and beyond—with ease.
Learn More AboutMirum Access Plus

