The Only IBAT Inhibitor
Available as an Oral
Solution and Tablet1

Simple Dosing
A once-daily medicine for cholestatic pruritus in patients with Alagille syndrome who are ≥3 months of age1
LIVMARLI oral solution
A dosing dispenser is provided to measure and deliver the prescribed dose accurately.1*
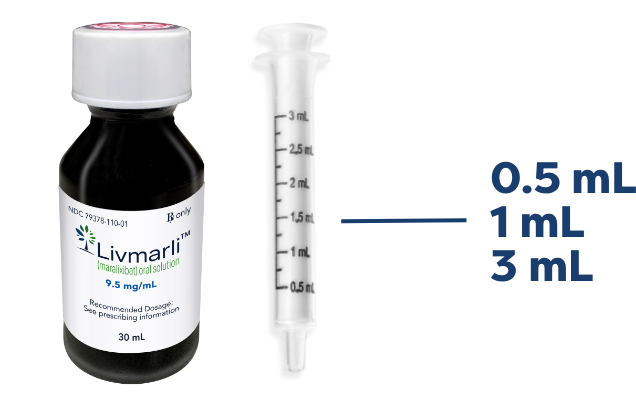

Clear, colorless to yellow grape-flavored liquid.1
LIVMARLI bottle and syringe not actual size.

LIVMARLI tablets
Tablets can be used in patients ≥25 kg who are able
to swallow them. They are available in 4 strengths1:

Tablets and dime not actual size.
Dime shown for scale.
One Dose,
Once Per Day
Both LIVMARLI oral solution and tablets should be taken once daily 30 minutes before a meal in the morning at the dosage prescribed1
If a patient misses a dose of LIVMARLI1:
- Advise them it should be taken as soon as possible if within 12 hours of the time it is usually taken. They can then resume their original dosing schedule
- If a dose is missed by more than 12 hours, the dose can be omitted and the original dosing schedule resumed
*Please advise patients that household teaspoons and tablespoons are not adequate measuring devices.2
IBAT=ileal bile acid transporter.
Prescribing LIVMARLI
To prescribe LIVMARLI for appropriate patients, download and fill out the Patient Enrollment form, and refer to the charts below for patient dosing details.
Download thePatient Enrollment Form
Once-Daily Dosing
The recommended dosage of LIVMARLI is 380 mcg/kg administered orally (PO) once daily (QD), taken 30 minutes before a meal in the morning1
Daily dose volume and Rx quantity by patient weight for LIVMARLI 9.5 mg/mL oral solution in patients with Alagille syndrome1
| Patient Weight (kg) | Days 1 to 7(190 mcg/kg once daily) | Beginning Day 8(380 mcg/kg once daily) |
| Volume QD (mL) | Volume QD (mL) | |
| 5 to 6 | 0.1 | 0.2 |
| 7 to 9 | 0.15 | 0.3 |
| 10 to 12 | 0.2 | 0.45 |
| 13 to 15 | 0.3 | 0.6 |
| 16 to 19 | 0.35 | 0.7 |
| 20 to 24 | 0.45 | 0.9 |
| 25 to 29 | 0.5 | 1 |
| 30 to 34 | 0.6 | 1.25 |
| 35 to 39 | 0.7 | 1.5 |
| 40 to 49 | 0.9 | 1.75 |
| 50 to 59 | 1 | 2.25 |
| 60 to 69 | 1.25 | 2.5 |
| 70 or higher | 1.5 | 3 |
Dose by patient weight for LIVMARLI tablets for patients with Alagille syndrome1†
| Patient Weight (kg) | Days 1 to 7(190 mcg/kg once daily) | Beginning Day 8(380 mcg/kg once daily) |
| Less than 25 | Use Oral Solution | Use Oral Solution |
| 25 to 32 | 10 mg | |
| 33 to 43 | 15 mg | |
| 44 to 65 | 10 mg | 20 mg |
| 66 or higher | 15 mg | 30 mg |
†Select the appropriate product based on the patient's weight and ability to swallow tablets.
Start dosing at 190 mcg/kg orally once daily; after 1 week, increase to
380 mcg/kg once daily, as tolerated1
The maximum daily dose is 28.5 mg (3 mL) per day for LIVMARLI oral solution and
30 mg per day for LIVMARLI tablets1

Tips for patients taking oral solution
- Be mindful of placement. Use the measuring device that comes with LIVMARLI to squirt the medicine into the inside of the cheek
- Add a flavorful twist. Suggest patients suck on fruit, such as an orange or lemon, before or after taking LIVMARLI
- Cool it. Once opened, consider storing LIVMARLI in the refrigerator3
Reminder: Advise patients to discard any remaining LIVMARLI oral solution 100 days after first opening the bottle.
Help Your Patients
Get Started
Provide your patients with the Patient Brochure to help them get to know LIVMARLI.

Pediatric patients aged 3 months to <12 months had similar safety, tolerability, and pharmacokinetic profiles to those ≥1 year old.1
Root Out
Excess Bile
Learn how LIVMARLI—the first FDA-approved treatment for cholestatic pruritus in Alagille syndrome—battles bile acid buildup.1
See How LIVMARLI Works
Encourage patients to download the Itch✓ app to help them track symptom patterns over time and generate customized reports to share at appointments.
Check Out the Itch✓ App
Mirum Access Plus assists both you and your patients at every turn, helping you navigate the payer approval process—and beyond—with ease.
Learn More AboutMirum Access Plus

