Improvements in
Cholestatic Pruritus

Cholestatic Pruritus Efficacy
Meaningful Improvements in Cholestatic Pruritus Were Seen as Early as 2 Weeks and Sustained Through 2 Years1-3
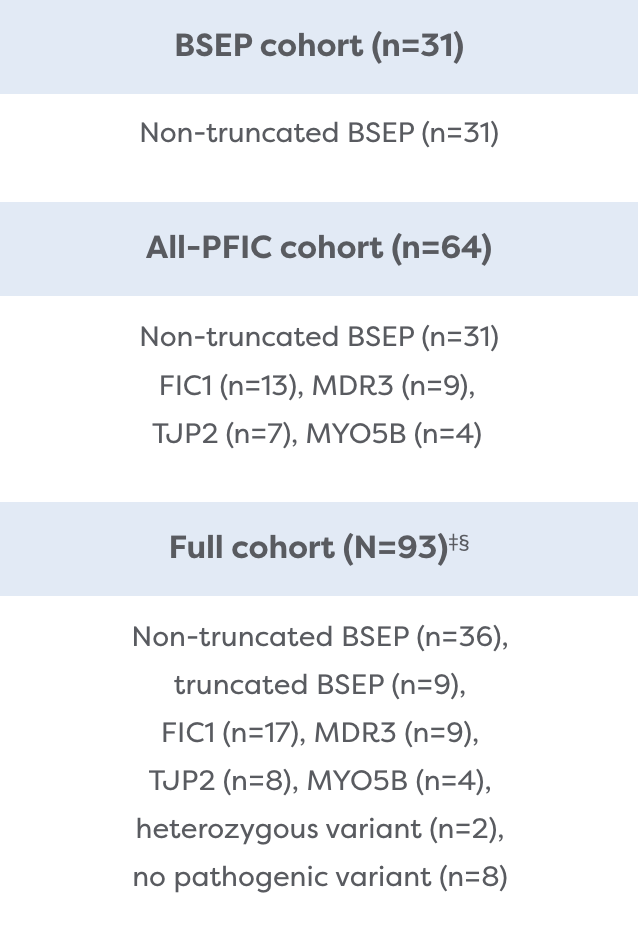
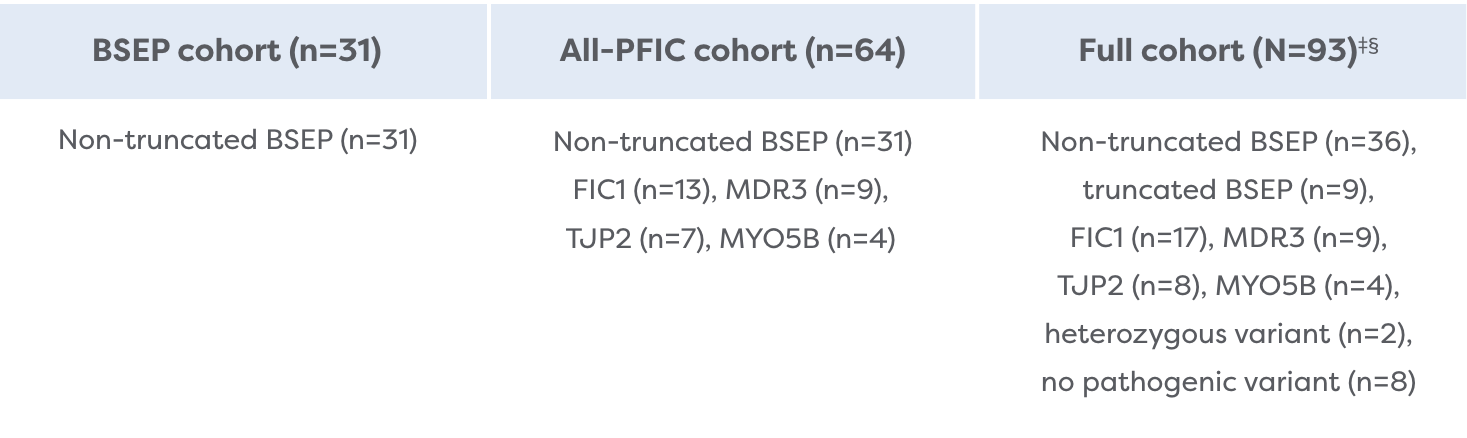
LIVMARLI was studied in the broadest population of progressive familial intrahepatic cholestasis (PFIC) subtypes and was shown to provide statistically significant improvements in cholestatic pruritus vs placebo at 6 months. Improvements were seen as early as Week 2.1,2,4
(ItchRO[Obs])
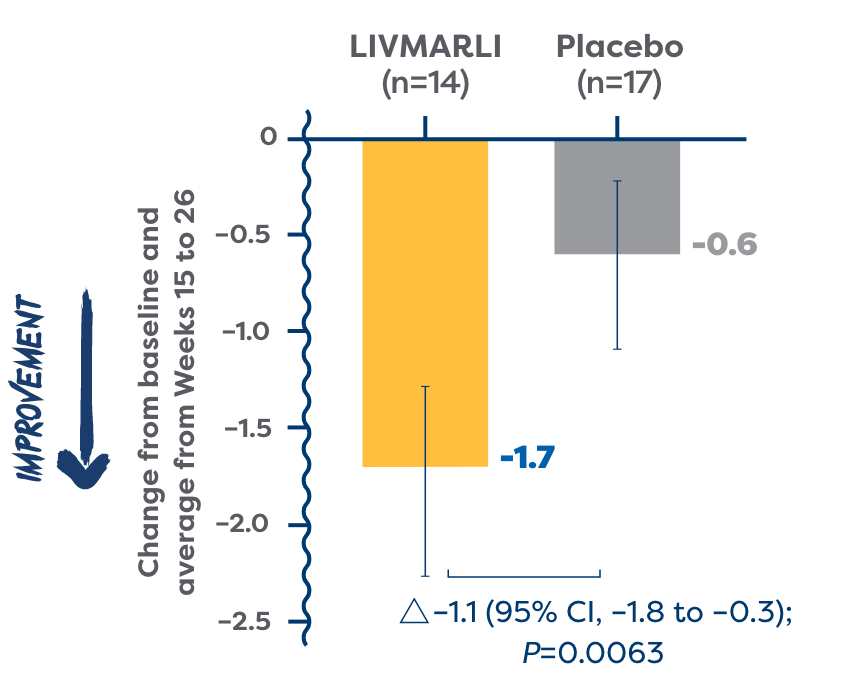
(ItchRO[Obs]) over time
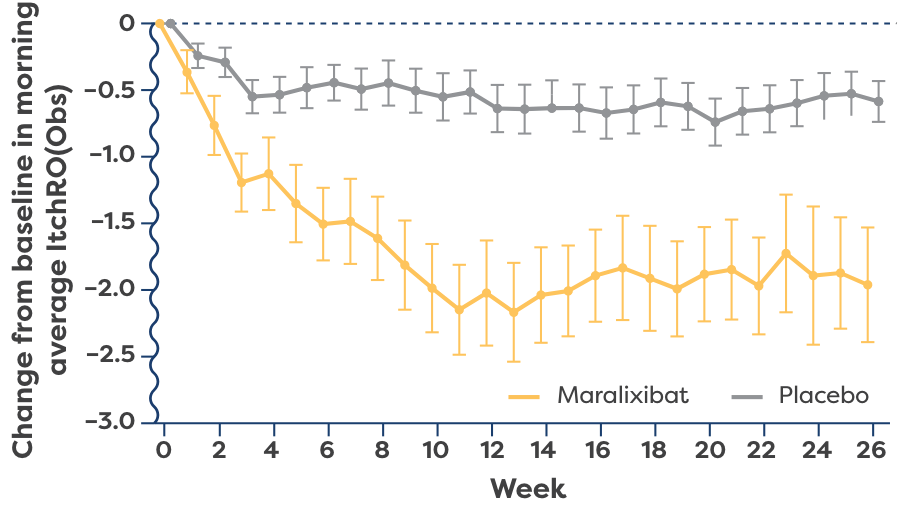
BSEP=bile salt export pump; ItchRO(Obs)=Itch Reported Outcome (Observer).
See Study Design >(ItchRO[Obs])
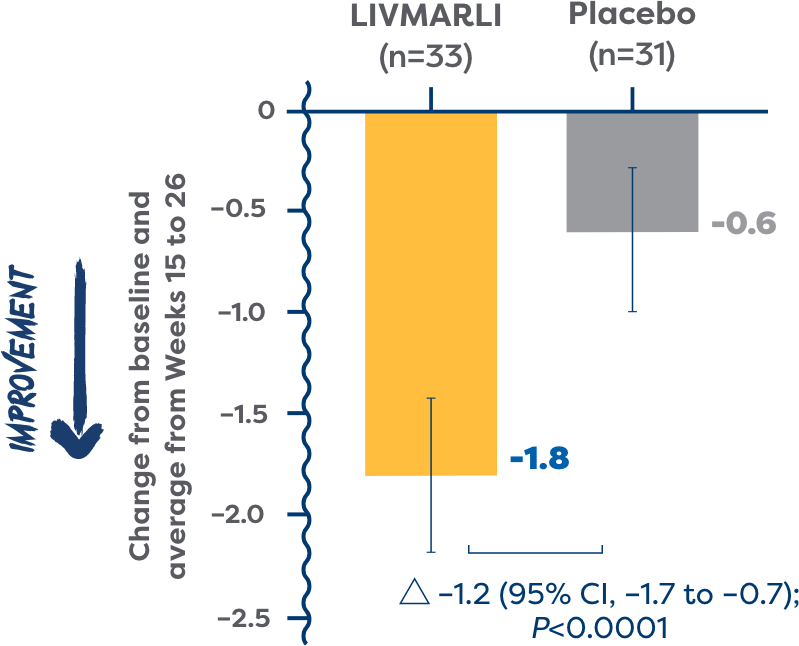
(ItchRO[Obs]) over time
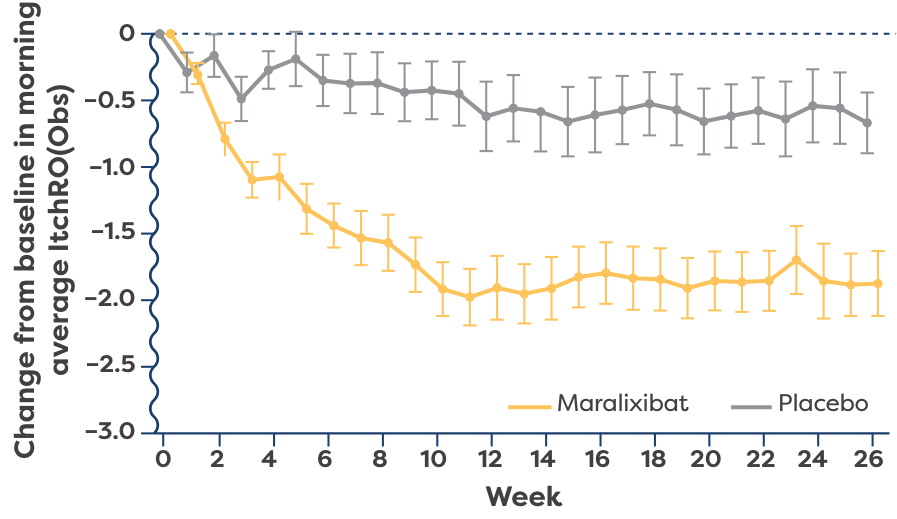
the first 6 months
of treatment
of patients in the All-PFIC cohort were considered cholestatic pruritus responders and experienced clinically meaningful improvements in cholestatic pruritus vs 29% of patients treated with placebo (P=0.0064).2
- Cholestatic pruritus responders had a ≥1-point ItchRO(Obs) improvement or a score of ≤1.02
no cholestatic pruritus
Over the 6-month period, patients treated with LIVMARLI (n=33) had significantly more days with little to no cholestatic pruritus than those who received placebo (n=31) (62% vs 28%, least squares [LS] mean difference 0.34, P<0.0001)5
Improvements Sustained Through 2 Years
In patients who remained on LIVMARLI (n=13) during the open-label extension, statistically significant improvements in cholestatic pruritus from baseline were sustained through 2 years (P<0.001).3
Improvements in Serum Bile Acid Levels
of patients treated with LIVMARLI in the All-PFIC cohort were considered serum bile acid (sBA) responders vs 7% of patients treated with placebo (P=0.0003).2
- sBA responders were defined as patients who achieved a ≥75% reduction in sBA levels or sBA <102 µmol/L2
≥75% reduction or sBA <102 µmol/L
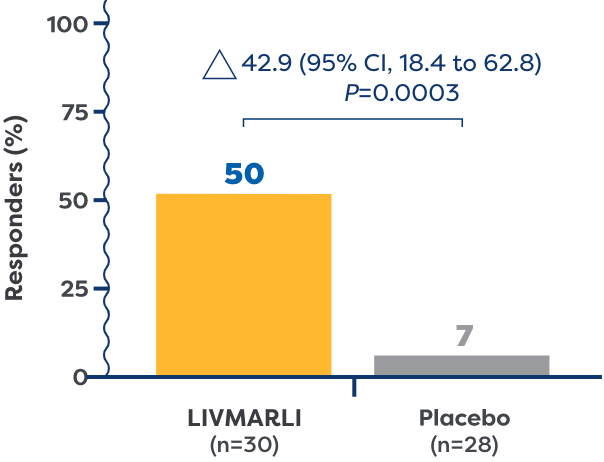
In the All-PFIC cohort, patients taking LIVMARLI saw statistically significant improvements in sBA levels compared with placebo (–157 µmol/L vs 3 µmol/L, LS mean difference –160 µmol/L, P<0.0001).2
Study Design
The Broadest Population of PFIC Subtypes Studied
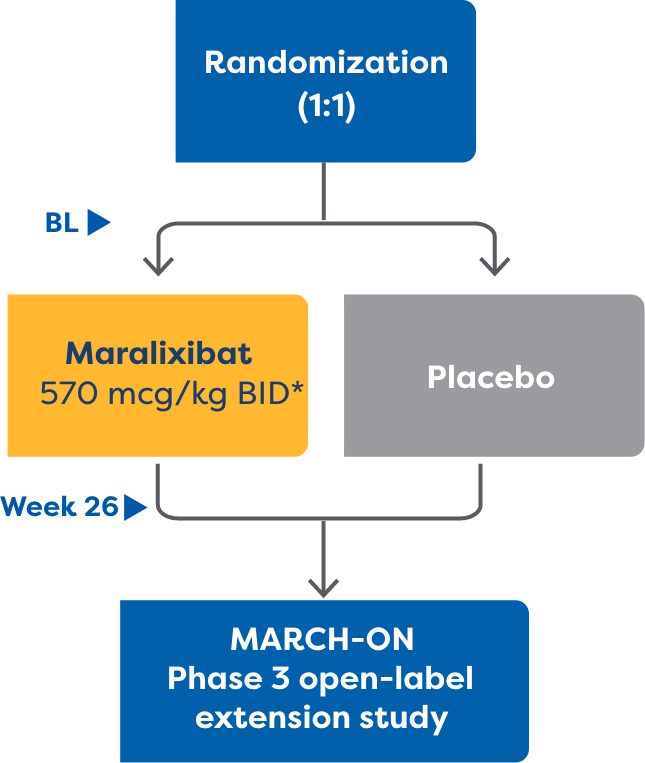
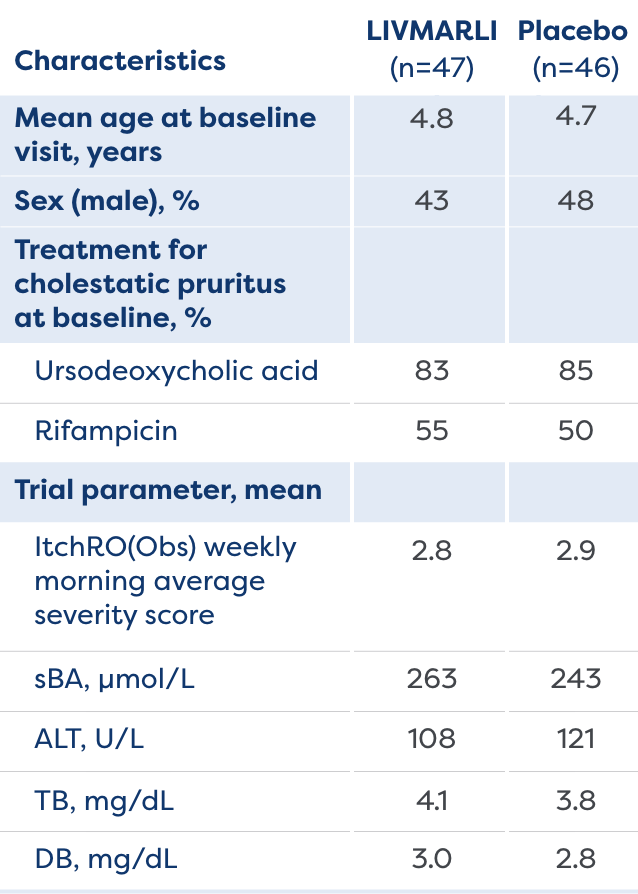
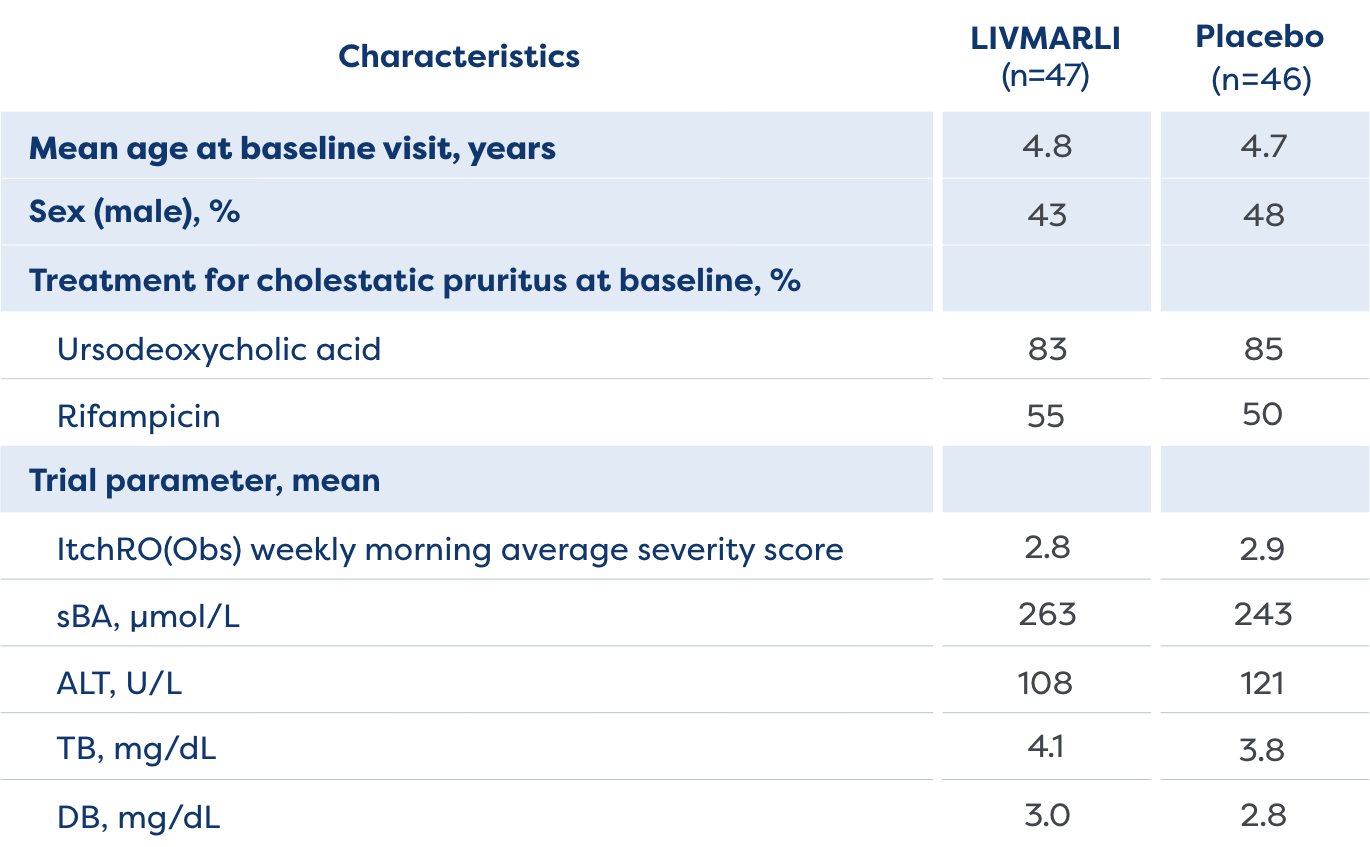
The MARCH-PFIC study was a 26-week, Phase 3, randomized, placebo-controlled study that assessed efficacy and safety of treatment with LIVMARLI in patients 12 months to <18 years old with cholestatic pruritus in PFIC.1,2
View Study Design and Baseline Characteristics

- Cholestatic pruritus
- sBA levels
- Bilirubin
- Growth Z-scores
- Incidence of treatment-emergent adverse events
Cholestatic pruritus was assessed at baseline using the Itch Reported Outcome (ItchRO) scale. ItchRO is a 0 to 4 scale, where 0 is none and 4 is very severe. This scale takes itch-related symptoms into consideration, including patients’ skin damage, sleep, and irritability.6,7
- Participants’ baseline scores were calculated as the average of the daily ItchRO scores over the 2 consecutive weeks during the screening period7
ALT=alanine transaminase; BL=baseline; BSEP=bile salt export pump; DB=direct bilirubin; FIC1=familial intrahepatic cholestasis associated protein 1; LS=least squares; MMRM=mixed model for repeated measures; MDR3=multidrug resistance class III; MYO5B=myosin VB; nt=non-truncated mutations; t=truncated mutations; TB=total bilirubin; TJP2=tight junction protein 2.
*In the dose escalation period, the dose was increased weekly starting at 142.5 mcg/kg twice daily to a maximum dose of 570 mcg/kg twice daily.2
†Endpoints were analyzed using a repeated measures model (MMRM) considering data from all study visits.1,2
‡Limitations of Use: LIVMARLI is not recommended in a subgroup of PFIC type 2 patients with specific ABCB11 variants resulting in nonfunctional or complete absence of bile salt export pump (BSEP) protein.1
§The full study cohort included 8 patients with prior surgery to treat PFIC. Surgery participants had the following PFIC types: non-truncated BSEP (LIVMARLI: 3; placebo: 0), FIC1 (LIVMARLI: 2; placebo: 2), and TJP2 (LIVMARLI: 0; placebo: 1).2
MARCH-PFIC is the largest Phase 3 study conducted in children with PFIC. It included PFIC types that had not previously been studied.2,4
Always Safety First!
LIVMARLI has a well-characterized safety and tolerability profile for cholestatic pruritus in patients with PFIC.1,2
See the Safety ProfileConvenient
Dosing
LIVMARLI is a liquid medication that is administered twice daily orally, 30 minutes before a meal.1
Look IntoLIVMARLI Dosing

Mirum Access Plus assists both you and your patients at every turn, helping you navigate the payer approval process—and beyond—with ease.
Learn More AboutMirum Access Plus

