Among 38 pediatric patients with PFIC who underwent surgical biliary diversion, the most common indication was cholestatic pruritus (92%).18
UNDERSTANDING PFIC
Bile Acid Buildup May Fuel Cholestatic Pruritus* Now and Impact the Liver Later1,2
Mechanism of Disease
Progressive Familial Intrahepatic Cholestasis (PFIC) Is a Group of Rare, Autosomal Recessive Disorders3
PFIC is classified into 3 main subtypes, with PFIC2 (bile salt export pump [BSEP] deficiency) being the most aggressive subtype and accounting for approximately half of cases.3-5 Across all PFIC subtypes, there is an inhibition of bile flow between the liver and small intestine, resulting in a persistent state of cholestasis.6,7
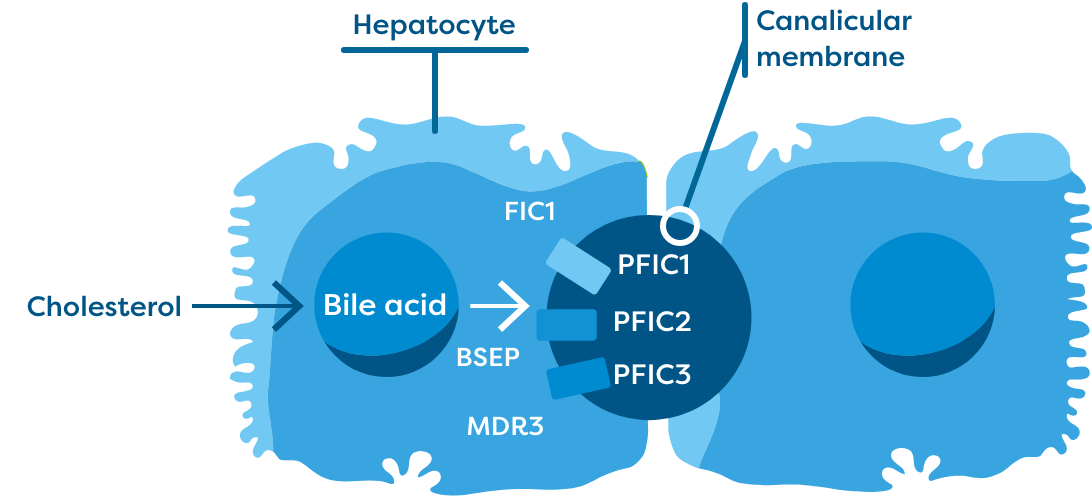
The 3 most common subtypes of PFIC (ie, PFIC1, PFIC2, and PFIC3) present with elevated sBA—nearly 10- to 20-fold elevations—with extremely elevated levels in PFIC211-13
Mutations in hepatocellular transport genes cause functional deficiencies that disrupt the ability for bile acids to be transported out of hepatocytes. This results in increased serum bile acid (sBA) levels systemically.3,8-10
Consequences of Cholestasis
Cholestasis leads to bile acid buildup in the liver and is the main driver of 1,7,14,15:
Inflammation
Progressive liver injury
Fibrosis
In PFIC1 and PFIC2, it has been reported that approximately
50%
of patients are alive and have their native liver at 10 years old.16
- In PFIC2, specifically, only 32% of patients are alive and have their native liver by 18 years old4


The Potential for Surgical Intervention and Liver Transplant
Many patients with PFIC with uncontrolled cholestatic pruritus continue to opt for surgical biliary diversion and/or liver transplant.4,7,17
End-stage liver disease (80%) was the most common indication for liver transplant in patients with PFIC, followed by refractory cholestatic pruritus (20%).19
End-stage
liver disease
Refractory cholestatic pruritus
For patients with PFIC, there is a need for effective treatment options that reduce bile acid buildup and promptly relieve cholestatic pruritus.7
BSEP=bile salt export pump; FIC1=familial intrahepatic cholestasis 1; MDR3=multidrug resistance class 3.
*The pathophysiology of cholestatic pruritus in PFIC is not completely known.

